While the cure rate of pediatric acute lymphoblastic leukemia (ALL) now exceeds ~90% with contemporary combination chemotherapy, the prognosis for adults with ALL remains significantly inferior with long-term overall survival ranging from 50% to 70%. Recent studies have uncovered marked differences in ALL genomics between children and adults, with some high-risk subtypes becoming more prevalent with age. However, the underlying biology of age-related disparities in ALL is not fully understood, especially with regard to differences in leukemia sensitivity to chemotherapy.
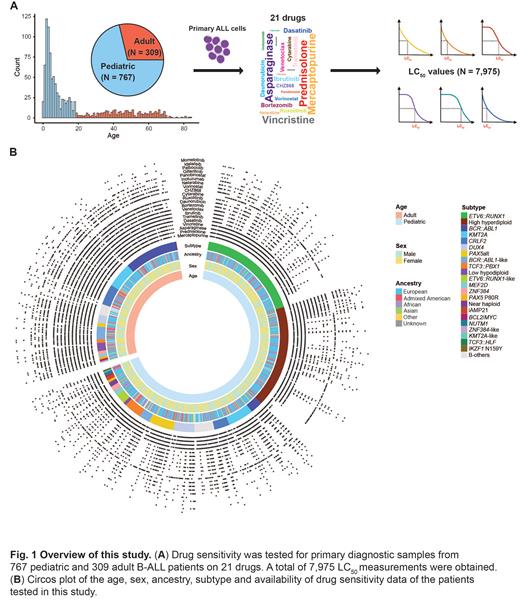
To address this knowledge gap, we performed ex vivo drug sensitivity profiling (i.e., pharmacotyping) of 21 anti-leukemia agents on primary B-ALL diagnostic samples from 767 pediatric (age, 0-18 years) and 309 adult (19-84 years) patients. Drug sensitivity was measured as LC 50: the concentration of drug required to kill 50% of the leukemia cells (PMID: 36604538). A total of 7,975 unique LC 50 values were experimentally determined. RNA-seq was used for subtype classification and gene expression analysis.
Among 21 drugs, seven showed significant differences in overall LC 50 between children and adults ( P<0.05 after Bonferroni correction): children displayed higher sensitivity to asparaginase, prednisolone, mercaptopurine, daunorubicin, and inotuzumab, while adults showed higher sensitivity to dasatinib and nelarabine. In multivariate models adjusting for 23 ALL molecular subtypes, only mercaptopurine remained significantly associated with age ( P=1.5×10 -5), suggesting that age-related differences in drug sensitivity can be primarily attributed to the variation in ALL subtypes between children and adults.
For mercaptopurine, within KMT2A, CRLF2, and DUX4 subtypes, pediatric samples consistently showed a lower LC 50 than adults carrying the same genomic abnormality ( P=0.032, 0.0045, and 0.02, respectively). To explore intra-subtype heterogeneity, we performed unsupervised clustering using gene expression data for each of these three subtypes. Remarkably, within each of these subtypes, we identified two clusters with distinct transcriptomic profiles that were also largely segregated by age group, i.e., an adult-dominated cluster (C-a) and a pediatric-dominated cluster (C-p). In the KMT2A subtype, cases in C-a exhibited an over-representation of the KMT2A:: AFF1 fusion, and resistance to mercaptopurine ( P=0.029), prednisolone ( P=0.0039), vincristine ( P=0.046) and cytarabine ( P=0.0037). Within CRLF2 ALL, cases in C-a were associated with the presence of BCR:: ABL1-like signature and IGH:: CRLF2 rearrangements, and were more resistant to mercaptopurine ( P=0.0073) and prednisolone ( P=0.00036) compared to those in C-p. For DUX4 ALL, C-a was characterized by an under-representation of ERG deletions and resistance to mercaptopurine ( P=0.0056) and prednisolone ( P=0.0031), compared to C-p within DUX4.
To explore the clinical relevance of this heterogeneity, we analyzed the in vivo treatment response of KMT2A (N=35), CRLF2 (N=59) and DUX4 (N=118) B-ALL enrolled in six frontline ALL trials. Compared to cases in C-p (usually drug-sensitive), those in C-a (usually drug-resistant) consistently had significantly poorer initial treatment responses as measured by persistent end-of-induction minimal residual disease (≥0.01%) in KMT2A (58% vs 9%; P=0.0063), CRLF2 (74% vs 41%; P=0.030), and DUX4 (66% vs 40%; P=0.0058) ALL.
In conclusion, these studies have revealed important new insights into the pharmacogenomic basis of age-related differences in B-ALL treatment response. These results indicate that both inter- and intra-subtype heterogeneity contribute to inferior prognosis in adults with ALL, but also point to therapeutic opportunities to improve their outcomes.
Disclosures
Mullighan:Illumina: Honoraria; Amgen: Honoraria; Pfizer: Research Funding; Abbvie: Research Funding. Relling:Servier: Consultancy. Jeha:Amgen: Other: As part of the mission of St. Jude Global Hiroto Inaba, Victor Santana, Sima Jeha and Caitlyn Duffy participate in the Blincyto Humanitarian Access Program and provide in kind support for this program. . Karol:Servier: Consultancy; Jazz Pharmaceuticals: Consultancy. Inaba:Servier: Consultancy; Amgen: Other: As part of the mission of St. Jude Global Hiroto Inaba, Victor Santana, Sima Jeha and Caitlyn Duffy participate in the Blincyto Humanitarian Access Program and provide in kind support for this program.. Konopleva:Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties. Jain:Newave: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novalgen: Research Funding; TransThera Sciences: Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; MEI Pharma: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Kite/Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Dialectic Therapeutics: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Aprea Therapeutics: Research Funding; CareDX: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Takeda: Research Funding; Servier: Research Funding; Fate Therapeutics: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Medisix: Research Funding; Mingsight: Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Incyte: Research Funding; BMS: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pfizer: Research Funding; Genentech: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Loxo Oncology: Research Funding; ADC Therapeutics: Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Ipsen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; Cellectis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses. Stock:Kite: Consultancy; Kura: Research Funding; Servier: Other: Data Safety Monitoring Board/Advisory Board; Newave: Honoraria; Amgen: Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Glaxo Smith Kline: Consultancy. Jabbour:Adaptive Biotech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Evans:BioSkryb Genomics: Membership on an entity's Board of Directors or advisory committees; Princess Maxima Centre for Childhood Cancer: Membership on an entity's Board of Directors or advisory committees. Yang:Takeda Pharmaceutical Company: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal